Glooko FDA Cleared to Market Its Diabetes Management Products
by GENE OSTROVSKY
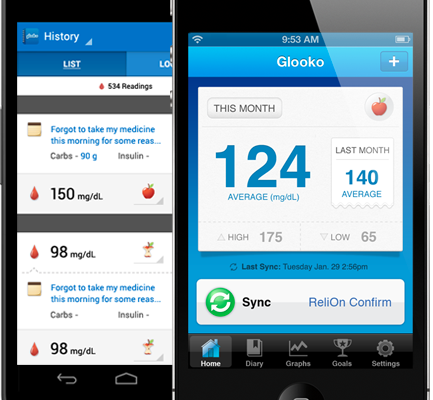
Glooko, Inc. of Palo Alto, CA was given clearance for its line of over-the-counter glucose management products that aim to simplify tracking of glucometer readings, food intake, and other relevant parameters.
The MeterSync Cable is used to connect any of 15 compatible glucometers to an iOS device for syncing readings with the Logbook and Logbook Charts apps.
Previously only available to users in the European Union, today also marks the debut of Glooko Logbook Charts in the U.S., which gives people using Glooko Logbook a more in-depth analysis and visualization of blood glucose data through easy to read charts that can be shared with healthcare professionals. The charts can be displayed in three formats: chart by time of day, chart by date, and chart by analysis of time of day. These charting capabilities give users and their healthcare team a comprehensive way to compare blood glucose readings over time so they can view how different factors—from diet and exercise to medication and treatments—impact their readings.
Here’s a quick look at the Glooko Logbook app:

Product page: Glooko Logbook Charts…
iTunes: Glooko Logbook…
Flashback: Glooko IR Adapter Connects Patients’ iPhones to Their Glucose Meters